In 2021,feellife is as a leader company of mesh nebulizer,invited to participate in the seminar on the finalization of the guidelines for the technical review of the registration of net nebulizer,as one of the three companies that participated in the seminar and revision of the guidelines.
The purpose of this guideline is to guide the registration applicant to submit the registration application materials of the net nebulizer, and also provide a reference for the technical review department to review the registration application materials.
Scope of application
The guideline applies to mesh nebulizers,which are active medical devices that convert medicinal liquids into aerosol particles through meshes,and are intended to be used to nebulize meshed liquid medicines for inhalation therapy by patients.
Registration Review Points
Regulatory Information
·product name requirement
·Calssification code
·Product list
·Principles and examples of registration unit division
·Principles and examples for determining typical products in the same registration unit
Summary data
·Structure and composition
The structure and composition of the product should be described according to the actual situation of the product, and the words 'main' and 'etc' should not be used for description.

·Working principle
According to the working principle, mesh nebulizers are generally divided into active mesh type and passive mesh type.
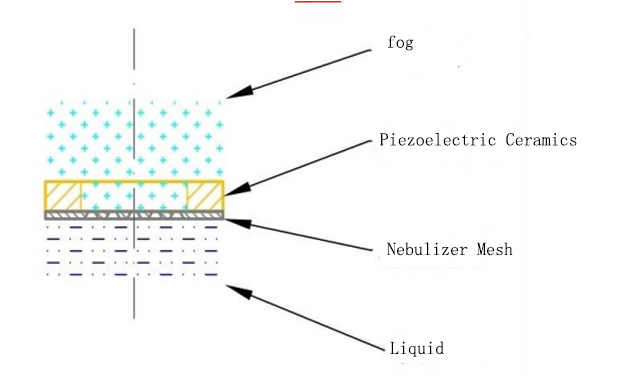
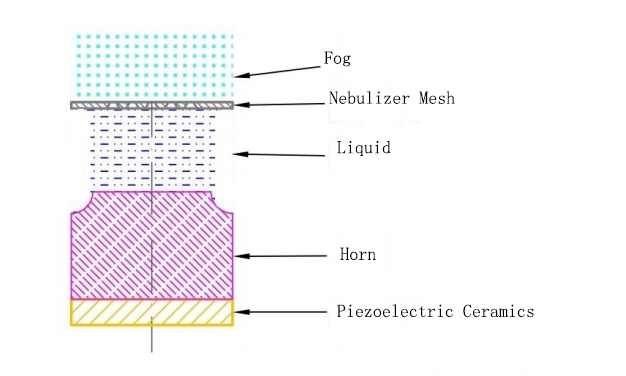
·Packaging description
·Scope of application/intended use of the product
·Contraindications
·Adverse event history for the product
Nonclinical data
·Product risk management information
·Product technical requirements
·Product research materials
Clinical evaluation data
Product Specifications and Label Samples
Quality management system documentation
