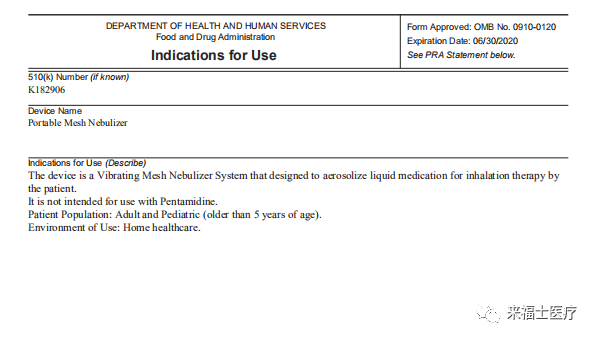
On September 28, Shenzhen feellife Medical Equipment Co., Ltd. was awarded FDA 510(K) certification, which is a major honorary qualification that feellife medical has obtained in 2020. This indicates that the feellife mesh nebulizer meets the FDA regulations and technical requirements, and has obtained a pass to enter the US market. This is bound to help make feellife medical to a broader stage.
FDA 510(K) (Food and Drug Administration) certification is the abbreviation of U.S. Food and Drug Administration. It is an international medical review authority authorized by the U.S. Congress, the federal government, specializing in food and drug The highest law enforcement agency for drug administration. According to the different risk levels, FDA divides medical devices into three categories (Ⅰ, Ⅱ, Ⅲ), category Ⅲ risk, high level; a small number of category I, III, most of the medical devices of category II, sold in the United States, need to do 'products' Listing Registration' (PMN: Premarket Notification) certification. The documents to be submitted for 'product listing registration' must meet Chapter 510 of the US FD&C Act, so certifications such as 'product listing registration' are often referred to as 510(K) certification.
Foods, drugs, cosmetics and medical devices certified by the FDA are safe and effective for the human body. In nearly 100 countries such as the United States, only FDA-approved materials, devices and technologies can be used for commercial clinical applications.
Due to its scientific nature and rigor, this certification has become a world-recognized standard.
feellife nebulizing Medicine Co., Ltd. FDA certification
feellife is an international medical enterprise integrating the research and development, production and sales of portable micro-mesh nebulizers. It was selected as a national high-tech enterprise in 2016, aiming to provide a third way of drug delivery for humans. The company is headquartered in Shenzhen, and has production plants in Los Angeles, Shenzhen Songgang, Shenzhen Huangtian and Huizhou respectively.
Up to now, feellife has researched and invented surge, frequency conversion atomization technology and a patent group with Mesh V+ as the core, mastered the core technology of atomization industry 'two titanium and one core', and passed domestic CFDA and international CE, FDA, ISO and other standard certifications , to create a smart, portable, healthy and safe mesh atomizer for users with ingenious craftsmanship. At the same time, the company has linked digital cloud technology to create a one-stop hospital solution for the Respiratory Cinema APP, bringing blessings to the healthy life of human beings. Therefore, the company's products are sold in more than 50 countries and regions at home and abroad, and are deeply trusted and loved by consumers around the world.

In the future, feellife will continue to hold high the banner of technological innovation, increase technological investment, further strengthen technological innovation capabilities, and continuously enhance the core competitiveness of the company, providing global users with portable micro-mesh fog with leading technology, reliable quality, and easy-to-use products. chemicalizer. Protect the respiratory health of the Chinese people and continue to fight for the cause of human respiratory health.